PORTLAND, Ore. (KOIN) — Painkillers are being recalled across the United States after a manufacturer’s mislabeling has led to a risk that some patients could be taking double what they were prescribed, according to an alert sent Thursday from the Food and Drug Administration.
The FDA says Bryant Ranch Prepack Inc. released one lot of 30-milligram tablets and one lot of 60-milligram tablets whose labels were reversed in error. The company issued the recall alert voluntarily on Wednesday.
Some patients who were prescribed the 30-milligram pills could be taking far more than they think they are as the bottles may actually contain double the dosage, the FDA says. They are at risk of overdose and death. Those prescribed the 60 milligram pills could feel withdrawal and pain as those bottles may have 30-milligram pills inside, the recall alert says.
Officials said there have not been any reports of adverse effects, but any who experience them can report it to the FDA online here.
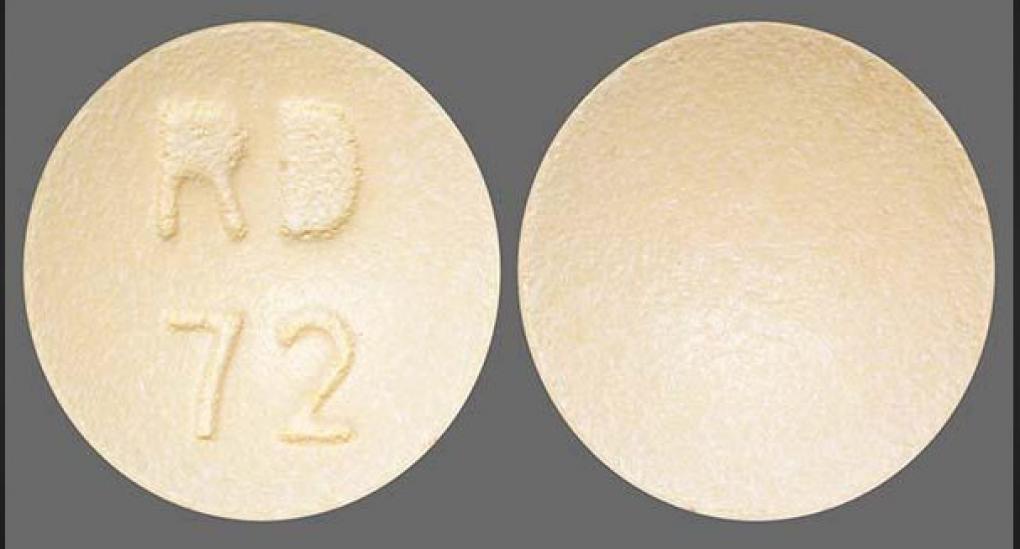
The 30-milligram pills affected are round, purple-colored film-coated tablets with “RD” and “71” etched into one side. The 60-milligram pills are round, light orange and film-coated, with “RD” and “72” visible on one side.
The pills have been sent across the country in bottles of 100 tablets each.
Every patient prescribed the painkillers is urged to stop using them immediately and contact the manufacturer at cs@brppharma.com or call 877-885-0882.