PORTLAND, Ore. (KOIN) — With the U.S. on track to authorize a second COVID-19 vaccine by the end of the year, some may confuse the unprecedented speed of the vaccine’s development with recklessness.
But Oregon State University Professor of Pharmacy Gaurav Sahay said that fear couldn’t be further from the truth. Sahay said this vaccine is backed by science and decades of work developing mRNA technology, which is his specialty.
“I hear people say things like ‘oh, I don’t want to be the guinea pig and be the first to get it, so I’ll wait,'” said Sahay. But he said the general public shouldn’t see themselves as test subjects — that honor goes to the 70,000 brave men and women who volunteered to be in the vaccine trials. Thanks to them, the vaccine has been proven to be 95% effective in protecting people from the novel coronavirus.
There were many crucial findings that helped Moderna and Pfizer develop a vaccine in under a year.
Initial research suggests there are at least six strains of the virus that cause COVID-19 but the mutation rate is low, making it easier to develop a vaccine.
The COVID-19 infection rate and the immediate economic impact that shut down the world certainly helped accelerate the vaccine’s development.
Also important to note is that both COVID-19 vaccines not only went through three routine clinical trials, but those trials saw impressive rates of participation. Vaccine trials are typically lucky to have 5,000 participants; Pfizer’s trial garnered more than 43,000 participants with data showing more than 94% efficacy with not serious side effects. Moderna’s trial had nearly 28,000 people.
“Like all clinical trials, these were conducted as phase one, phase two, phase three,” Sahay said. “The only thing some of the trials were conducted in parallel. Manufacturing was started while waiting. Usually, industry doesn’t start manufacturing before phase three comes in but because of this pandemic all those kinds of things were happening.”
Dr. Joe Sullivan, an Oregon Health Authority senior health advisor, said the reason most vaccines take such a long time to develop is due to a lack of funding to conduct trials. The speed in developing the COVID-19 vaccine is largely due to funding from Operation Warp Speed.
Corners weren’t cut and roadblocks weren’t removed. It’s important to distinguish the difference.
“I think the reward is just outstanding at this point because as you look at that 94% efficacy — when we started, the FDA had said that it should be like 50%,” Sahay said. “So the benefits are clear.”
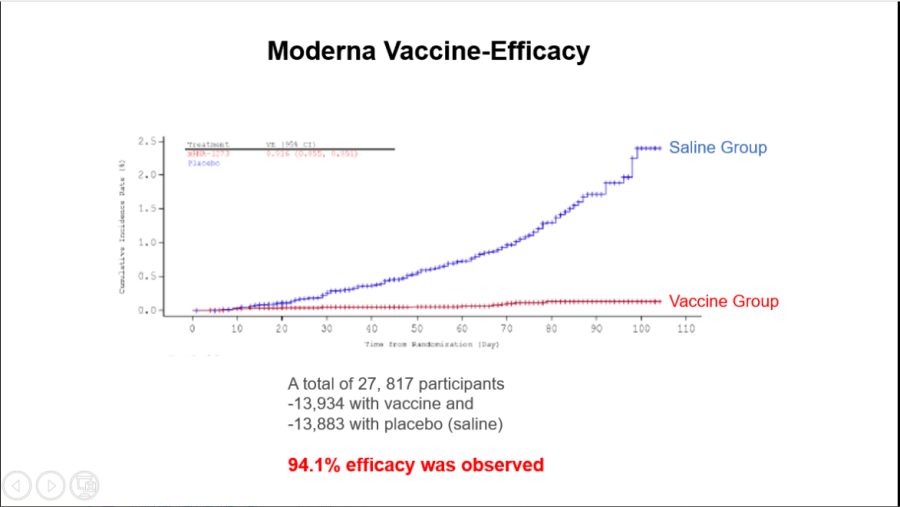
Moderna COVID-19 efficacy results. 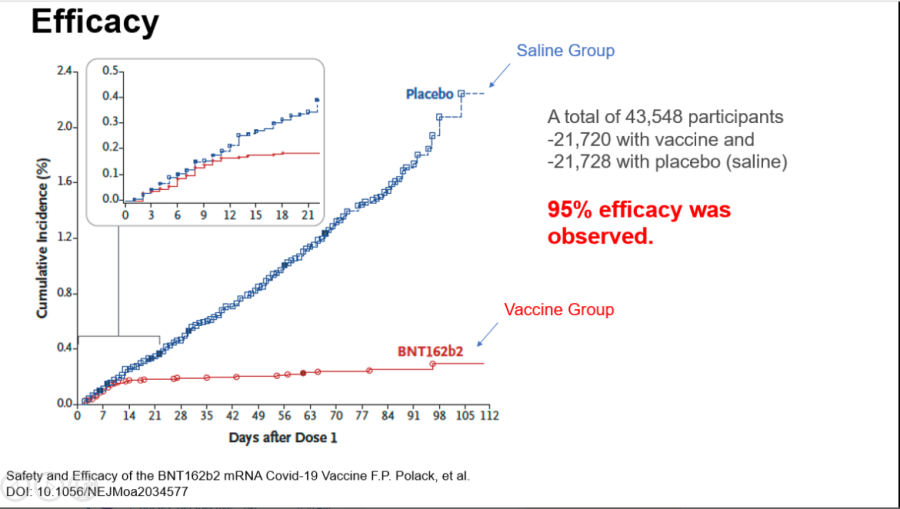
Pfizer COVID-19 efficacy results.
Sahay weighed the real-life scenarios for the risk and rewards for getting the vaccine. The FDA reports trials found recipients experienced some mild symptoms like muscle soreness, fatigue and headaches. The FDA advises doctors not to administer the Pfizer vaccine to individuals with known severe allergic reactions to components within the vaccine.
Read more on the FDA’s guidance
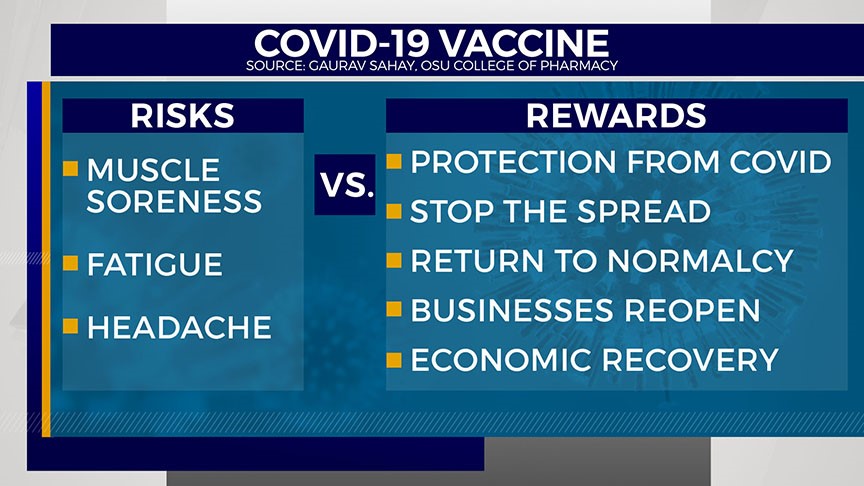
In terms of rewards, Sahay underscored protection from COVID-19, stopping the spread, returning to normal social life, businesses possibly reopening and the economy moving toward recovery.
But to reach these rewards, scientists say about 75% of the population will need to get vaccinated to achieve herd immunity.
“This is the only way we will get out of this pandemic,” said Sahay. “I’m just waiting to get the vaccine myself and for my family.”
With 330 million people in the United States, roughly 250 million Americans will need two doses of the vaccine. Until then, masks, mandates and closures will continue.
“Stay patient. But this is the beginning of the end,” said Sahay.