PORTLAND, Ore. (KOIN) — A recall has been issued for insulin pens from one manufacturer because they may not have labels on the injectors, the Food and Drug Administration said in a press release Thursday.
The manufacturer, Mylan Pharmaceuticals Inc., issued the voluntary nationwide recall, which affects one batch of unbranded U-100, 3-milliliter insulin glargine-yfgn pens, Batch No. BF21002895.
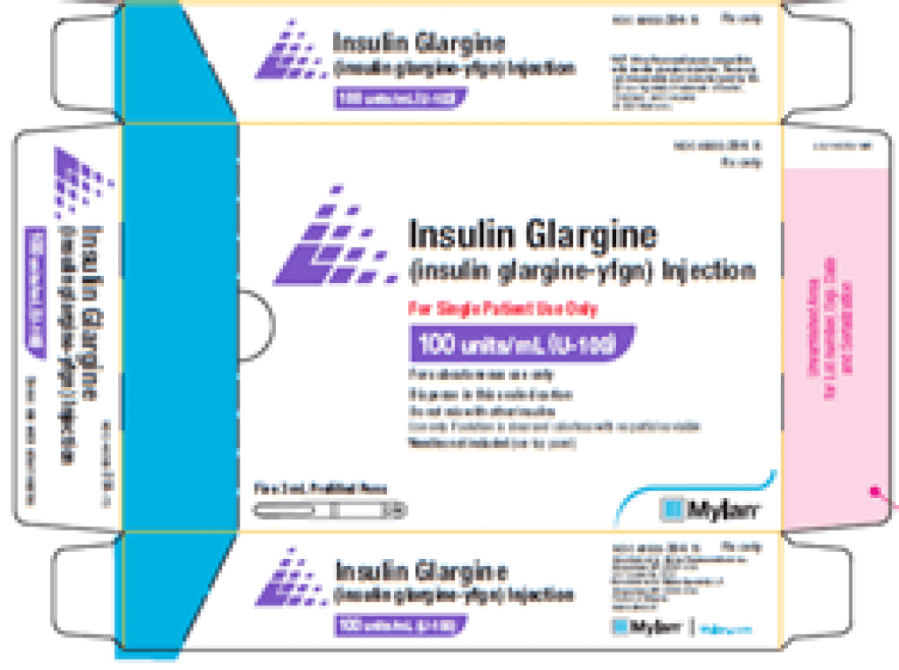
FDA officials say missing labels could present some problems for patients trying to properly monitor their blood sugar levels.
As of Thursday, the FDA said there have been no reported adverse reactions from the public. Adverse reactions from the product can be reported to the FDA here.
Officials told anyone having issues as a result of taking the recalled diabetes medication to contact their health care provider. Anyone with questions about the recall can also contact the manufacturer’s parent company, Viatris, at 800-796-9526 or customer.service@viatris.com.
The recall only applies to the manufacturer’s unbranded insulin glargine-yfgn pens and not the Semglee brand units.
Click here for more information on the recall.
