PORTLAND, Ore. (KOIN) – Where did all the snow go? We know snow melts when it’s too warm but sometimes it seems like it just vanishes without leaving any puddles behind. How is that possible? Let’s discuss!

First, as you may know, there are three phases of water: liquid, solid and gas. Examples include raindrops, ice, and water vapor.
Something occurs when you go from one of the three states to the other state. In this weather lesson, we are going to focus on the process of transitioning from a solid straight to a gas.
Wait, I thought a solid had to melt first before becoming a gas. Ah, the vapor caper is at it again, stealing any chance for a solid to become a liquid.
What is this process called?
SUBLIMATION: This is the process of turning ice straight into a gas without going through the phase of melting. You may hear this called the solid-vapor transition.
Check out the graphic below:
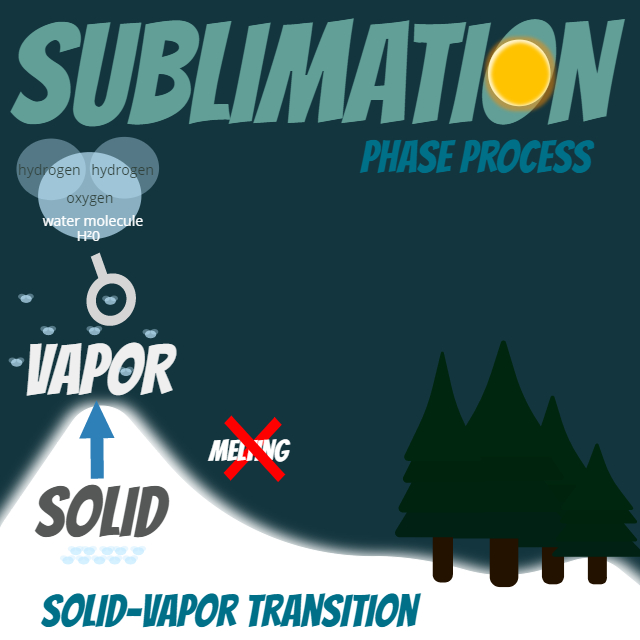
Now that you have an idea of what the name of the process is, you should go ahead and see it in action.
WATCH THE VIDEO ABOVE NOW


How does this process work? Well we need a little energy or heat to get this going. You may notice the sun in the graphic. That is going to be the aid to the process. Water takes a certain level of energy to convert that solid directly to a gas. Some other ingredients that help the process, low temperatures, windy conditions and low air pressure. Where do we find all of these? Well up on the mountain is a good start.
EXTRA

Above is an image of dry ice and you can see the process of sublimation taking place. Dry ice is a solid which sublimates and turns to gas at -78.5 °C (-109.3°F).
If you’re interested in all of the phase process, make sure to check out this weather kids article
